novavax vaccino covid
Those vaccines give genetic instructions to the cell on how to make a piece of the spike protein that is unique to the virus that causes COVID-19. Novavax and the National Research Council NRC continue to work toward production of a COVID-19 vaccine in Canada according to a statement from the industry ministers press secretary John.

New Novavax Vaccine High Protection Against Covidnew Novavax Vaccine High Protection Against Covid Insurance Italy
This new vaccine may cause fewer side effects than those currently available in the US.

. Novavax COVID-19 Vaccine Nuvaxovid Covovax NVX-CoV2373 Indication Nuvaxovid NVX-CoV2373 is a vaccine candidate indicated as a SARS-CoV-2 coronavirus vaccine to prevent COVID-19. Early last month the pharmaceutical company Novavax shared that its two-dose COVID-19 vaccine was more than 90 effective at preventing COVID-19. The technology binds with human receptors targeted by virus which is critical. Get the latest facts and information from the Government of Canada.
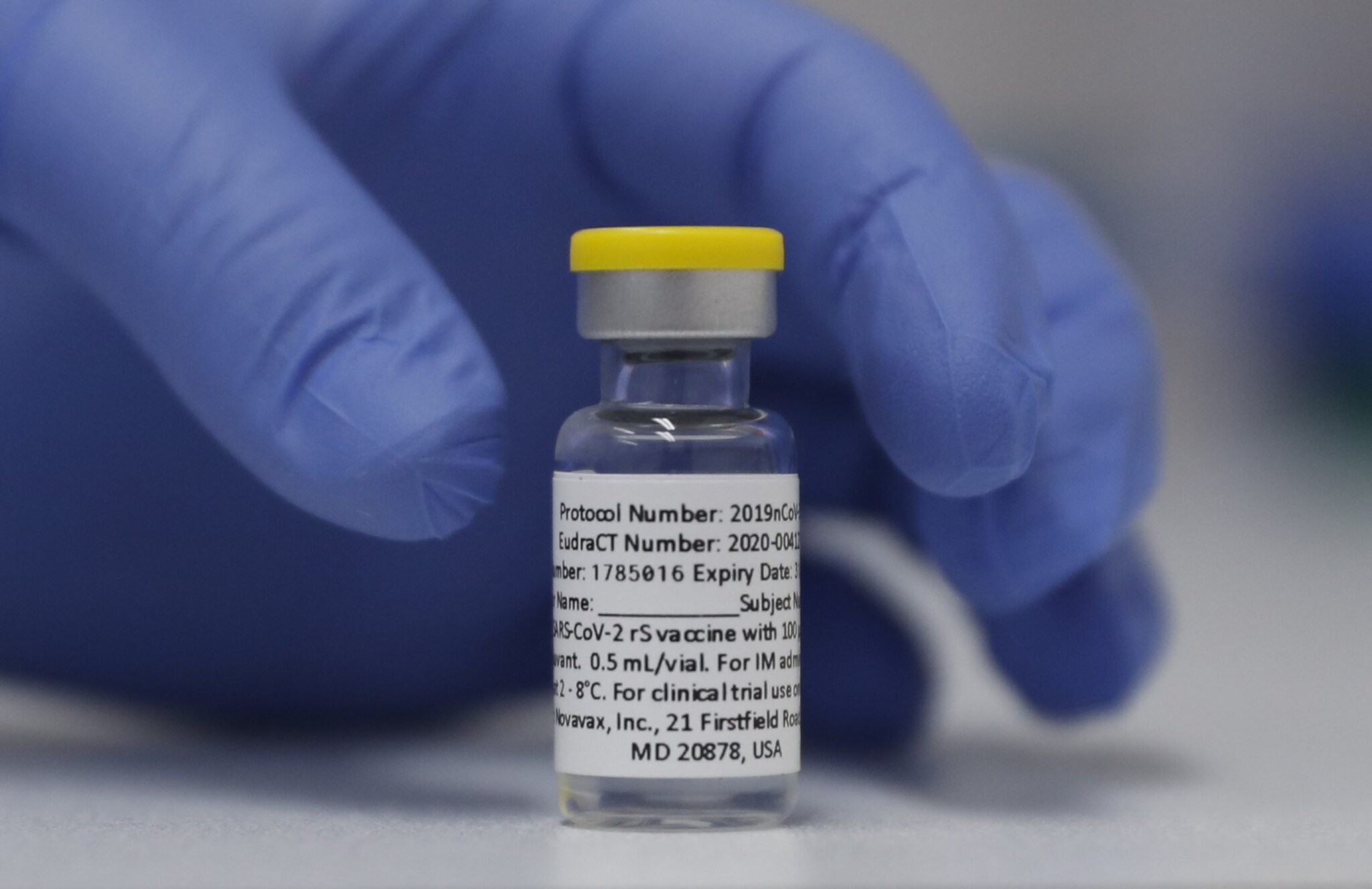
Still a dead protein-based COVID-19 vaccine isnt a sure winner in Europe Betsch says. Novavax COVID-19 vaccine is packaged as a ready-to-use liquid formulation in a vial containing ten doses. The latest Novavax data confirm that its possible to achieve the same efficacy against COVID-19 with a more familiar technology that more people may be inclined to trust. The Novavax COVID vaccine also looks like it performs well.
Novavaxs NVAX filing with the FDA seeking approval for emergency use authorization of its COVID-19 vaccine NVX-CoV2373 gets further postponed to January 2022. The mRNA vaccines. The company releasing data that says that its- like many of its competitors its booster shot as part of a. Authorized Use of Novavax Covid-19 Vaccine in the Philippines The Philippines Food and Drug Administration has issued Emergency Use Authorization EUA for Covovax Recombinant Spike Protein of SARS-CoV-2 Virus 5 mcg for active immunization of individual 18 years of age and older for the prevention of coronavirus disease 2019 caused by SARS-CoV-2.
Ad Everyone living in Canada will have access to free safe effective COVID-19 vaccines. Novavaxs vaccine uses moth cells to produce the coronavirus spike protein then combines it with an immune-boosting soaplike substance called an adjuvant. The Novavax coronavirus vaccine is engineered from the genetic sequence of coronavirus with nanoparticle technology. The Maryland-based company Novavax has developed a protein-based coronavirus vaccine called NVX-CoV2373In March the company announced an efficacy rate of 96 percent against the original.
The vaccine is derived from the spike protein pulled from the very first strain of the novel coronavirus and is bolstered by an adjuvant or. In phase 3 trials the final phase of testing in humans it was 90 protective against developing symptomatic COVID with no severe cases. The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix-M adjuvant given intramuscularly 21 days apart. The vaccine is stored at 2- 8 Celsius enabling the use of existing vaccine supply and cold chain channels.
NVX-CoV2373 demonstrated efficient binding with receptors targeted by the virus in preclinical trials a critical aspect for effective vaccine protection. Ad Everyone living in Canada will have access to free safe effective COVID-19 vaccines. Maryland-based Novavax says its two-dose vaccine. COVID-19 vaccines train the body to recognize the virus by spotting the spike protein that coats it but the Novavax option is made very differently than.
Reuters - Novavaxs COVID-19 vaccine could receive approval from Europes drug regulator next week and subsequently an emergency use listing from the World Health Organization the Financial. It was an impressive feat for a company that had never brought a product to market and did not own a single manufacturing facility at the time. Ad Follow the roll-out of COVID-19 vaccination in Quebec. Get the latest facts and information from the Government of Canada.
But experts have pointed to an additional interesting tidbit in the research. Vaccine safety efficacy side effects. And one of them has to do with the vaccine developer Novavax. In summer 2020 Novavax was awarded 16bn to produce up to 100 million doses by Operation Warp Speed the US government programme to expedite covid vaccines.
Novavax CEO says COVID-19 vaccine will roll out very quickly Novavax President and CEO Stanley Erck on its vaccine being 904 effective in a clinical trial and why the booster shot will be its. Dopo che lente regolatore europeo per i medicinali Ema ha ufficialmente approvato il vaccino anti Covid Novavax è arrivato il via libera anche dalla Commissione europea all.

Vaccino Novavax Perche E Diverso Dagli Altri E Come Puo Aiutarci Contro Il Covid La Repubblica

Corona Vakzin Von Novavax Kein Totimpfstoff Experte Platzt Der Kragen Panorama

Johnson Johnson Aktuell News Zum Corona Impfstoff Faz

Novavax Autorizzato Da Ema Arriva Il Via Libera E Il Quinto Vaccino Anti Covid Approvato In Europa Il Mattino It
Komentar
Posting Komentar